


|
|
 |
|
|
 |
 |
|
|
      |
A Basic Introduction What is Cooling with Electrons? "Hot" and "cold" are words we use to describe the presence (or absence) of heat. Heat is best described as energy contained within something else. So a cup of hot coffee has more energy than that same cup an hour later, after much of the heat has dissipated. The energy which makes up "heat" is the kinetic energy of the atoms which carry the heat. So if the atoms in the cup of coffee are very active, the coffee is "hot". If the atoms become less active, the coffee is "cold". And if the atoms get cold enough so that the atoms are no longer in a fluid form, the coffee freezes into a solid. While atoms in a solid themselves tend to be pretty immobile, the sub-atomic particles within them are always moving. At any temperature above absolute zero, electrons are constantly in motion, spinning around the atom, but also (especially in metals) swapping places with the electrons of surrounding atoms. Of course, some electrons have high energy, while some electrons have low energy. The low energy electrons are cold, while the high energy electrons are hot. Cooling with electrons involves encouraging the high energy electrons to escape, bringing in low energy electrons to replace them. It is analogous to removing the loudest people from a party: the party gets quieter. What makes Cool Chips special? There are other technologies which use electron migration to reduce heat. These fall under the rubric of "thermoelectrics". These technologies all use special materials and geometries to move the hottest electrons to one side, keeping the coldest electrons at the other. The biggest problem with thermoelectrics is that while electrons are used to carry heat in one direction, the material itself returns most of that heat through conduction! Cool Chips are special because the electrons move across a gap -- and that gap, since it is not a solid, is an excellent insulator. Once heat is trapped on one side, it cannot easily return. 
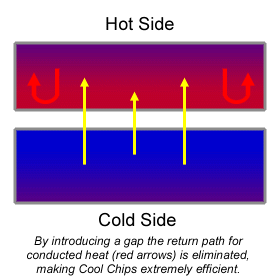
How do we get the electrons to move across the gap? The difficulty in getting lots of electrons to flow across a gap is that electrons do not naturally leave their atoms to go into space. Electrons do jump around a lot (it is called tunneling), but those jumps are pretty short, from one to ten nanometers, or just a few billionths of a meter long.
Researchers at Cool Chips plc have figured out how to get electrons to move off a surface more easily;
called the Avto Metal technology, materials emit electrons
more easily than they otherwise would.
With the addition of a voltage bias, which encourages the electrons to move in a given direction, the heat
is then transferred from one side to the other. And because there is a gap between the two materials, the
heat cannot simply flow back!
Once these devices become commercially available, they will not only revolutionize the industries of refrigeration and cooling, but all of those industries that depend on them. Copyright © 1995 - 2012 Cool Chips plc. All rights reserved. Forward Looking Statement may be found at http://www.coolchips.gi/fwdlook.shtml. |